Chemical Reaction: Is a change of one or more substances into a different substance(s)
How do we know when a chemical reaction takes place? What type of evidence would show a chemical reaction has occurred?
- Temperature change
- Emission of Light
- Formation of a gas
- Color change
- Formation of a solid
- Smell change
Do chemical reactions happen in your everyday life? If so give an example.
What happens when you leave your bike out in the rain?
When you cut up an apple and leave it sitting out, what do you observe?
Are you growing taller?
Procedure
1. Students work in pairs. For each pair of students provide the following:
A small clear plastic labeled cup with baking soda (NaHCO3)
A small clear plastic labeled cup with calcium chloride (CaCl2)
One quart size zip lock bag
Graduated cylinder
A 250-mL beaker containing 150 mL of phenol red – phenol red is an indicator which is red in neutral solution and yellow in acidic solution
2. Have students make observations of the calcium chloride (CaCl2), baking soda (NaHCO3), and phenol red solution. Have them discuss and share their observations.
3. In a 1 quart zip lock bag have students add:
1 level spoonful of NaHCO3 and 2 level spoonfuls of CaCl2.
4. Show students the graduated cylinder. Ask them what it is. What is it used for? Students pour 25 mL of phenol red solution from the beaker into the graduated cylinder (to the black mark).
5. Collect the solid material in one corner of the bag and twist the bag so that the solid is in its own compartment.
6. Pour 25 mL of phenol red solution (phenol red and water) into the bag so that it does not come in contact with the solids. One student holds the bag (keeping the solid material in its own compartment), while the other student adds the phenol red.

7. Seal the bag.
8. Shake the bag allowing the solid and the liquid to mix.
9. Discuss and share observations.
10. Set aside the bag containing the solution. Keep it sealed to observe any changes at the end.
11. How do we know a chemical reaction occurred?
12. What is the gas produced in the reaction?
13. What gas in the air do we breathe in that we need? O2 What do we breathe out? CO2
14. What is dry ice? CO2 (s)
15. Put some dry ice into a ziplock bag containing some phenol red solution? Seal the bag? What do you observe? Has a chemical reaction occurred? What is the evidence for a chemical reaction?
The change in color from red to yellow shows the solution has turned acidic. Do you know a food that is acidic? Lemons, limes, generally anything that tastes sour is acidic. CO2 in the air is absorbed in water producing carbonic acid, causing the water to become acidic. Do you think the fish, plants and coral reefs can survive in water that is more acidic?
THE BROADER PICTURE
Use the graph below to answer the following questions about carbon dioxide.
Carbon Dioxide Levels in the Atmosphere
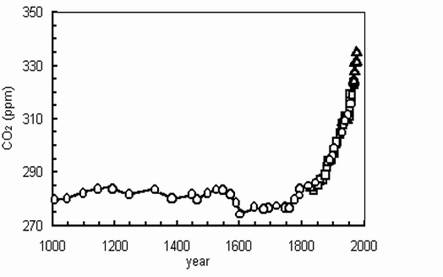
How does the level of carbon dioxide change over time?
What is the cause for the increase in CO2 in the atmosphere? Human activity: The Industrial Revolution – we build factories, car exhaust, etc. Is it a problem to have lots of CO2 in the atmosphere? Yes, global warming, acidification of oceans and lakes.
CENSURF: One of the goals of our new Center for Sustainability (CenSURF) at UCSB is to convert atmospheric CO2 into usable carbon-based compounds.
Observations: You set aside the bag containing the calcium chloride, baking soda and phenol red solution. Have any changes occurred? Discuss your observations of any changes that occurred? Initially the solids dissolved and CO2 gas was produced. Then CO2 was absorbed into the solution forming a new solid formed. This is what happens in the atmosphere as well. We produce CO2, the CO2 is absorbed in oceans and lakes and we observe new solids being formed, CaCO3(s), chalk or limestone.
 Exploring chemical reactions and their role in our environment. Presented by CenSURF (The Center for Sustainable Use of Renewable Feedstocks, an NSF Center for Chemical Innovation) Exploring chemical reactions and their role in our environment. Presented by CenSURF (The Center for Sustainable Use of Renewable Feedstocks, an NSF Center for Chemical Innovation)
|

