

| VRML Player | Download Cortona to view 3D models | Link |
| Ethylene | LUMO (Lowest-Energy Unoccupied Pi Orbital) | 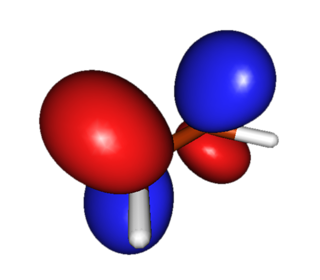 |
| Ethylene | HOMO (Highest-energy Occupied Pi Orbital) | 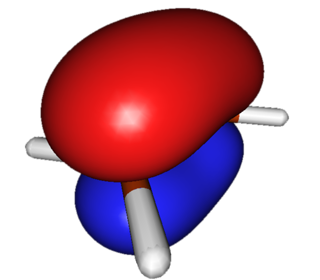 |
| Enolate |
HOMO Highest occupied Pi molecular orbital in enolates is delocalized between the carbonyl oxygen and α carbon. Both sites can act as bases but the carbon center is a better nucleophile and most interesting chemistry involves formation of bonds via α carbon. |
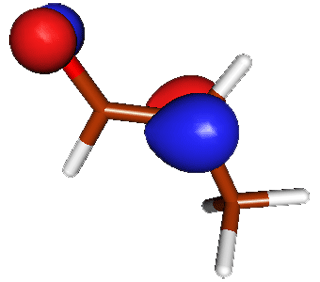 |
| Methoxy Butadiene | Electrostatic Potential Mapped onto Electron Density. | 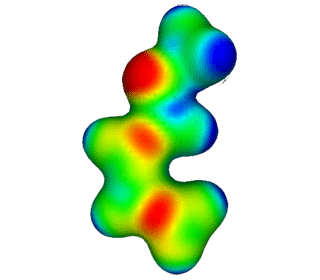 |
| Anisole |
HOMO Highest occupied Pi molecular orbital in anisole. |
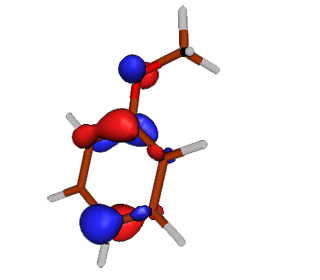 |
| Anisole | Electrostatic Potential Mapped onto Electron Density. | 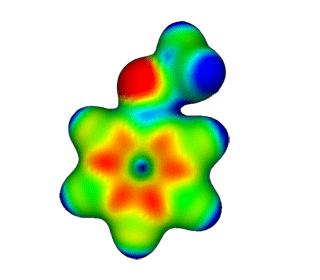 |
| Aniline | Electrostatic Potential Mapped onto Electron Density. | 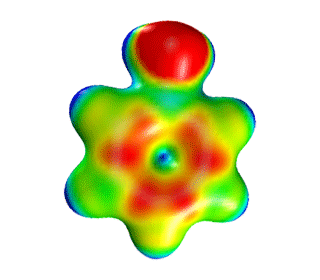 |
| Nitrobenzene | HOMO | 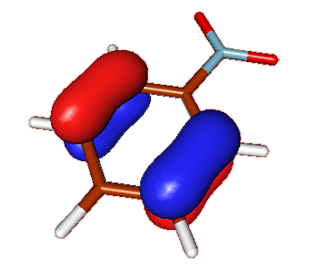 |
| Nitrobenzene | Electrostatic Potential Mapped onto Electron Density. | 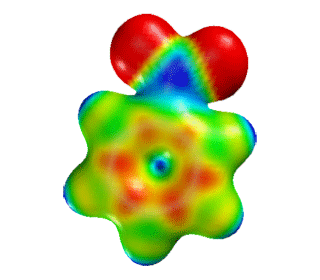 |
| Fluorobenzene | Electrostatic Potential Mapped onto Electron Density. | 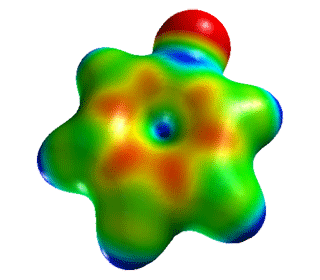 |
| Chlorobenzene | Electrostatic Potential Mapped onto Electron Density. | 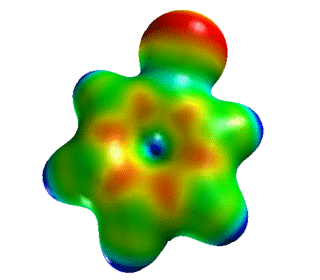 |