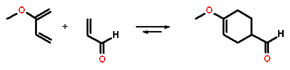Molecular Orbitals in Conjugated Systems
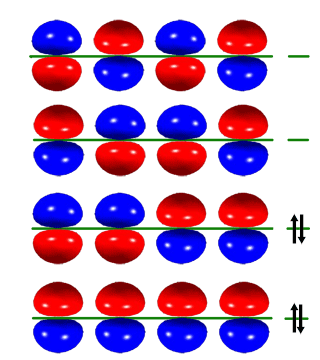 According to the frontier orbital theory, the chemistry of conjugated π systems is largely determined by the HOMO and LUMO π orbitals in the reactant molecules. The outcome of reactions involving interaction of π orbitals can be rationalized using the concepts of orbital phase and orbital symmetry. The figure on the right illustrates what is meant by the orbital phase using 1,3-butadiene as an example. In this molecule, four atomic p orbitals form four π molecular orbitals. The four molecular orbitals differ by the extent of favorable overlap, and thus in energy. The lowest energy MO forms by the in-phase overlap of all four p atomic orbitals; the next one forms when two pairs of in-phase atomic orbitals overlap; the third when one pair of in-phase atomic orbitals overlaps, and the highest energy molecular orbital forms when there are no in-phase overlaps. The MO's are filled with electrons starting with the lowest-energy orbital such that two electrons occupy an MO. In the case of 1,3-butadiene, there are 4 π electrons, thus two orbitals get filled. The second lowest-energy orbital is the HOMO.
According to the frontier orbital theory, the chemistry of conjugated π systems is largely determined by the HOMO and LUMO π orbitals in the reactant molecules. The outcome of reactions involving interaction of π orbitals can be rationalized using the concepts of orbital phase and orbital symmetry. The figure on the right illustrates what is meant by the orbital phase using 1,3-butadiene as an example. In this molecule, four atomic p orbitals form four π molecular orbitals. The four molecular orbitals differ by the extent of favorable overlap, and thus in energy. The lowest energy MO forms by the in-phase overlap of all four p atomic orbitals; the next one forms when two pairs of in-phase atomic orbitals overlap; the third when one pair of in-phase atomic orbitals overlaps, and the highest energy molecular orbital forms when there are no in-phase overlaps. The MO's are filled with electrons starting with the lowest-energy orbital such that two electrons occupy an MO. In the case of 1,3-butadiene, there are 4 π electrons, thus two orbitals get filled. The second lowest-energy orbital is the HOMO.
The Diels-Alder Reaction
The Diels-Alder reaction is a cycloaddition reaction between a conjugated diene and dienophile.
Diels-Alder reaction has high synthetic utility for making unsaturated six-membered rings. The reaction of 1,3-butadiene with unsubstituted dienophile (as shown above) is very slow (activation energy about 27 kcal/mol) but the Diels-Alder reactions occur readily when the alkene has an electron-withdrawing substituent. For example, acrolein is a good dienophile.
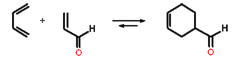
Cyclic alkenes, especially ones where the double bond is conjugated to a carbonyl, can be used as dienophiles. For example,
maleic anhydride is a very good dienophile.
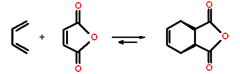
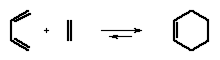
The diene is required to have an s-cis conformation. Cyclic dienes work well in this reaction. For example, a reaction between 1,3-cyclohexadiene and vinyl chloride yields a bicyclic reaction product.
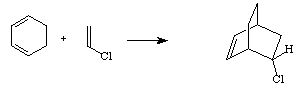
Note that chlorine is a relatively poor π-electron withdrawing group and the reaction above in not very fast. Interestingly, many Diels-Alder reactions occur much faster in water than in organic solvents. Scientists are still working on finding out why aqueous environment accelerates this reaction.
Molecular Orbitals in Diels-Alder Reaction
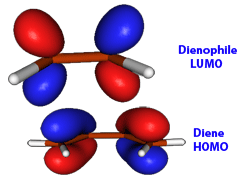 The details of the mechanism of Diels-Alder reaction reaction are somewhat debated but experimental and computational arguments suggest that the reaction with typical electron-rich dienes, and electron-poor dienophiles proceeds via asynchronous concerted mechanism. You can think of the electron-rich diene as a nucleophile with partially delocalized electrons. The electrons that matter most are on the Highest Occupied Molecular Orbital (HOMO) in the diene. For the bond formation to take place, these electrons shall "flow" into a Lowest Unoccupied Molecular Orbital (LUMO) in the dienophile, which at this stage acts as an electrophile.
The details of the mechanism of Diels-Alder reaction reaction are somewhat debated but experimental and computational arguments suggest that the reaction with typical electron-rich dienes, and electron-poor dienophiles proceeds via asynchronous concerted mechanism. You can think of the electron-rich diene as a nucleophile with partially delocalized electrons. The electrons that matter most are on the Highest Occupied Molecular Orbital (HOMO) in the diene. For the bond formation to take place, these electrons shall "flow" into a Lowest Unoccupied Molecular Orbital (LUMO) in the dienophile, which at this stage acts as an electrophile.
But the "flow" (or charge transfer) of electrons from diene to dienophile makes the dienophile more nucleophilic, and the diene, which has lost electrons, becomes at the same time more electrophilic. Thus, the electrons from HOMO of the dienophile can now "flow" to LUMO of diene; for the formation of the second bond the dienophile acts as a nucleophile. In the case of symmetric dienophiles, such as ethene or maleic anhydride, each end of the double bond can act with equal probability as the electrophilic site in the initial charge transfer from HOMO to LUMO. Furthermore, once the diene and the dienophile are properly positioned to allow for the charge transfer between the diene and the dienophile, the formation of two new sigma bonds occurs so rapidly that there are no zwitterionic or bi-radical intermediates. Thus, the Diels-Alder reaction is a concerted reaction.
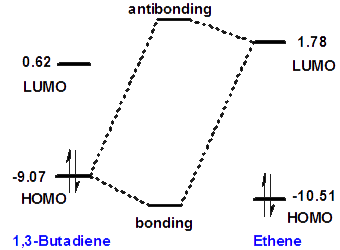 The orbital interactions in the Diels-Alder reaction can be also visualized as a diagram of orbital energies. The energy of HOMO can be estimated from the vertical ionization energy; the energy of LUMO corresponds to the electron affinity. Photo-electron ionization experiments reveal that ionization energies of 1,3-butadiene and ethene are 9.07 and 10.51 eV, respectively. Electron transmission spectroscopy reveal that electron affinities of 1,3-butadiene and ethene are -0.62 eV and -1.78 eV, respectively. The HOMO energies can also be obtained from quantum chemical calculations (8.6 eV and 10.4 eV for 1,3-butadiene and ethene, respectively) while the accurate calculation of LUMO energies is difficult. Based on these values, we can construct the orbital interaction energy diagram shown on the right.
The orbital interactions in the Diels-Alder reaction can be also visualized as a diagram of orbital energies. The energy of HOMO can be estimated from the vertical ionization energy; the energy of LUMO corresponds to the electron affinity. Photo-electron ionization experiments reveal that ionization energies of 1,3-butadiene and ethene are 9.07 and 10.51 eV, respectively. Electron transmission spectroscopy reveal that electron affinities of 1,3-butadiene and ethene are -0.62 eV and -1.78 eV, respectively. The HOMO energies can also be obtained from quantum chemical calculations (8.6 eV and 10.4 eV for 1,3-butadiene and ethene, respectively) while the accurate calculation of LUMO energies is difficult. Based on these values, we can construct the orbital interaction energy diagram shown on the right.
The diagram highlights the interaction between the HOMO of the diene and the LUMO of the dienophile, which leads to the formation of a new bonding molecular orbital between the two molecules. Analogously, the second interaction between the HOMO of ethene and LUMO of 1,3-butadiene (not shown) leads to the formation of the second bonding molecular orbital. These two bonding molecular orbitals become the two new sigma bonds in the product. The molecular orbital energies depend on the substituents in the diene and the dienophile. For example, the electron-withdrawing carbonyl substituent in acrolein significantly lowers the LUMO energy of the dienophile, bringing it closer to the HOMO energy of the diene. As a result, the energy of the new bonding orbital decreases in comparison with ethene. Acrolein is much more reactive than ethene in the Diels-Alder reaction.
The Diels-Alder reaction with asymmetric dienes and dienophiles usually show excellent regioselectivity: in the case of electron-rich dienes and electron-poor dienophiles, the most nucleophilic atom in the diene bonds to the most electrophilic atom in the diene.
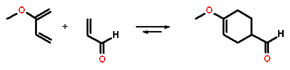
The Diels-Alder reaction is highly stereoselective: cis-substituted dienophiles yield cis-substituted cyclohexenes and trans-substituted dienophiles yield trans-substituted cyclohexenes. The stereoselectivity in Diels-Alder reaction can be rationalized considering the overlap of HOMO of one reactant with LUMO of the other.
Table below shows π molecular orbitals for ethylene (dienophile) and 1,3-butadiene; clicking on the image will bring up Virtual Reality Modeling Language models for orbitals.
| VRML Player |
Download Cortona to view 3D models
Download freeWRL to view 3D models |
Link
Link
|
| Dienophile |
LUMO (Lowest-Energy Unoccupied Pi Orbital). This orbital accepts electrons from the diene during the reaction. Electron-widtawing substituents conjugated to the double-bond reducing the Pi-electron density and allow for better "flow" of electrons to this orbital. In practice, alkenes with a conjugated carbonyl group are good dienophiles in the Diels-Alder reaction. |
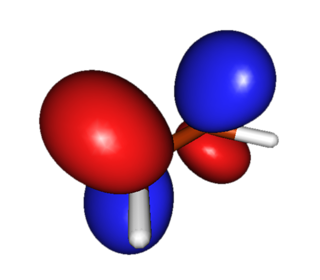 |
| Dienophile |
HOMO (Highest-energy Occupied Pi Orbital). This orbital reorganizes during the reaction via overlap with the LUMO of the diene. |
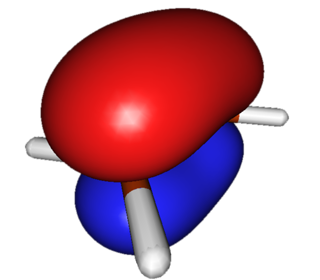 |
| Diene |
LUMO+1
High-energy unoccupied Pi molecular orbital in butadiene has three nodes and is asymmetric. This molecular orbital rearranges to become the asymmetric LUMO of the reaction product. |
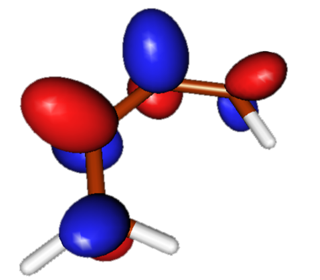 |
| Diene |
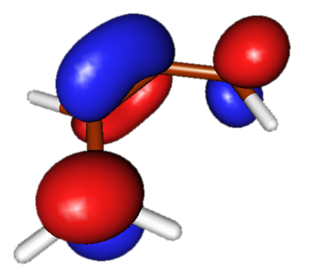
LUMO
Lowest unoccupied Pi molecular orbital in butadiene has two nodes and is symmetric. This orbital allows for a favorable overlap with symmetric HOMO of the dienophile during the reaction. |
 |
| Diene |
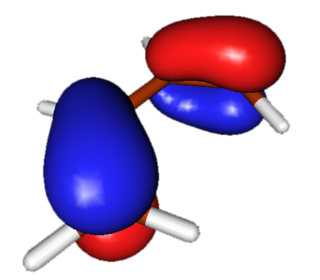
HOMO
Highest occupied Pi molecular orbital in butadiene has one node and is asymmetric. Electrons from this Pi orbital could "flow" to the antisymmetric LUMO of dienophile during the reaction, allowing for formation of a new carbon-carbon bond. |
 |
| Diene |
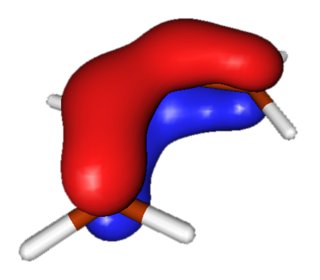
HOMO-1
Lowest-energy occupied Pi molecular orbital has no nodes and is symmetric. This molecular orbital rearranges to become the symmetric HOMO of the reaction product. |
 |
Outside Resources
Diels-Alder Reaction by Francis A. Carey at University of Calgary
Diels-Alder Reaction at www.organic-chemistry.org
Molecular Orbital Animation during Diels-Alder reaction by Jonathan Miller.
Synthesis of Limone via Diels-Alder Reaction by Alan Shusterman at Reed College.
Pericyclic Reaction Chemistry by Mark Leach at the Chermogenesis web book.
 According to the frontier orbital theory, the chemistry of conjugated π systems is largely determined by the HOMO and LUMO π orbitals in the reactant molecules. The outcome of reactions involving interaction of π orbitals can be rationalized using the concepts of orbital phase and orbital symmetry. The figure on the right illustrates what is meant by the orbital phase using 1,3-butadiene as an example. In this molecule, four atomic p orbitals form four π molecular orbitals. The four molecular orbitals differ by the extent of favorable overlap, and thus in energy. The lowest energy MO forms by the in-phase overlap of all four p atomic orbitals; the next one forms when two pairs of in-phase atomic orbitals overlap; the third when one pair of in-phase atomic orbitals overlaps, and the highest energy molecular orbital forms when there are no in-phase overlaps. The MO's are filled with electrons starting with the lowest-energy orbital such that two electrons occupy an MO. In the case of 1,3-butadiene, there are 4 π electrons, thus two orbitals get filled. The second lowest-energy orbital is the HOMO.
According to the frontier orbital theory, the chemistry of conjugated π systems is largely determined by the HOMO and LUMO π orbitals in the reactant molecules. The outcome of reactions involving interaction of π orbitals can be rationalized using the concepts of orbital phase and orbital symmetry. The figure on the right illustrates what is meant by the orbital phase using 1,3-butadiene as an example. In this molecule, four atomic p orbitals form four π molecular orbitals. The four molecular orbitals differ by the extent of favorable overlap, and thus in energy. The lowest energy MO forms by the in-phase overlap of all four p atomic orbitals; the next one forms when two pairs of in-phase atomic orbitals overlap; the third when one pair of in-phase atomic orbitals overlaps, and the highest energy molecular orbital forms when there are no in-phase overlaps. The MO's are filled with electrons starting with the lowest-energy orbital such that two electrons occupy an MO. In the case of 1,3-butadiene, there are 4 π electrons, thus two orbitals get filled. The second lowest-energy orbital is the HOMO. 

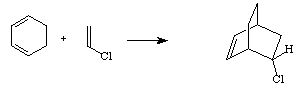
 The details of the mechanism of Diels-Alder reaction reaction are somewhat debated but experimental and computational arguments suggest that the reaction with typical electron-rich dienes, and electron-poor dienophiles proceeds via asynchronous concerted mechanism. You can think of the electron-rich diene as a nucleophile with partially delocalized electrons. The electrons that matter most are on the Highest Occupied Molecular Orbital (HOMO) in the diene. For the bond formation to take place, these electrons shall "flow" into a Lowest Unoccupied Molecular Orbital (LUMO) in the dienophile, which at this stage acts as an electrophile.
The details of the mechanism of Diels-Alder reaction reaction are somewhat debated but experimental and computational arguments suggest that the reaction with typical electron-rich dienes, and electron-poor dienophiles proceeds via asynchronous concerted mechanism. You can think of the electron-rich diene as a nucleophile with partially delocalized electrons. The electrons that matter most are on the Highest Occupied Molecular Orbital (HOMO) in the diene. For the bond formation to take place, these electrons shall "flow" into a Lowest Unoccupied Molecular Orbital (LUMO) in the dienophile, which at this stage acts as an electrophile.  The orbital interactions in the Diels-Alder reaction can be also visualized as a diagram of orbital energies. The energy of HOMO can be estimated from the vertical ionization energy; the energy of LUMO corresponds to the electron affinity. Photo-electron ionization experiments reveal that ionization energies of 1,3-butadiene and ethene are 9.07 and 10.51 eV, respectively. Electron transmission spectroscopy reveal that electron affinities of 1,3-butadiene and ethene are -0.62 eV and -1.78 eV, respectively. The HOMO energies can also be obtained from quantum chemical calculations (8.6 eV and 10.4 eV for 1,3-butadiene and ethene, respectively) while the accurate calculation of LUMO energies is difficult. Based on these values, we can construct the orbital interaction energy diagram shown on the right.
The orbital interactions in the Diels-Alder reaction can be also visualized as a diagram of orbital energies. The energy of HOMO can be estimated from the vertical ionization energy; the energy of LUMO corresponds to the electron affinity. Photo-electron ionization experiments reveal that ionization energies of 1,3-butadiene and ethene are 9.07 and 10.51 eV, respectively. Electron transmission spectroscopy reveal that electron affinities of 1,3-butadiene and ethene are -0.62 eV and -1.78 eV, respectively. The HOMO energies can also be obtained from quantum chemical calculations (8.6 eV and 10.4 eV for 1,3-butadiene and ethene, respectively) while the accurate calculation of LUMO energies is difficult. Based on these values, we can construct the orbital interaction energy diagram shown on the right.