Chem 142 Hmwk Set 2 Key
1-
The
characteristic pH at which the net electric charge is zero is the isoelectric
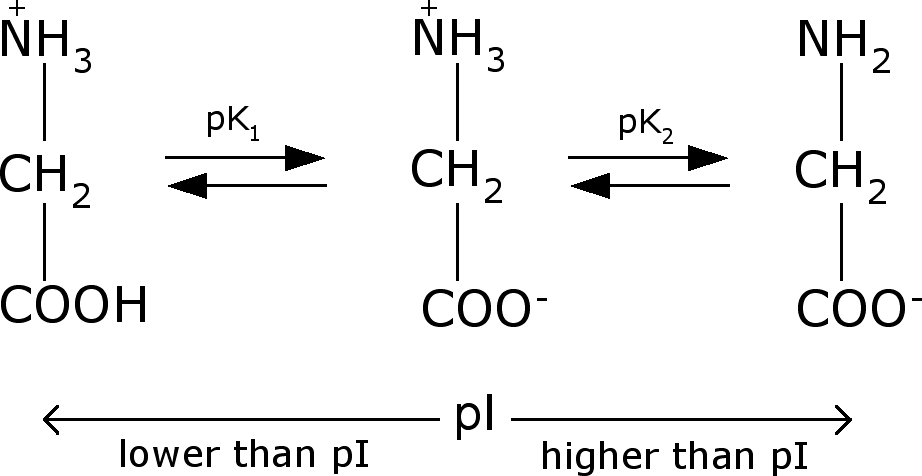
pH, designated as pI. Draw the structure of glycine at pH
i)
Well
below the pI
ii)
Well
above the pI
iii)
What is the
net charge of the amino acid at any pH below the pI.
The structure of glycine at low and high values of pH is shown below.
At a pH equal to the pI equimolar concentrations of positively and negatively
charged species are present. Thus
at its pI glycine, like all molecules, is neutral. At pH below the pI, the ÐCOO- group begins to
pick up a proton and the glycine is in its fully protonated, +1 charge state.
- What is
the difference between a protein and a polypeptide chain?
The word polypeptide refers to any polymer of peptide bond-linked (ie
amide-linked) alpha-amino carboxylic acids. The word protein denotes a subset of polypeptides. In general the word is used to imply
the subset of polypeptide that folds into a unique 3-dimensional structure that
is biologically functional (or was derived via mutation or modification of a
functional molecule).
- A measure
of the hydrophobicity of an amino acid is the free energy of transferring
it from water to, for example, olive oil. You have a test tube filled with an aqueous solution of
tryptophan. You pour oil over
the top of it and shake like crazy (to equilibrate it). How would you then determine the
free energy of transfer?
Because tryptophan has a relatively
hydrophobic, aromatic sidechain, the amino acid will migrate into the oil
until, if you shake long enough, there will be equilibrium between aqueous and
oil phases.
Trp (aq) ó Trp (in oil)
Equilibrium constant can be obtained from
the relative concentrations.
![]()
![]()
Thus, to determine DG
you simply measure this equilibrium constant. How do you do this?
You simply measure the concentration of tryptophan in each of the two
solutions and take the ratio. The
concentration of tryptophan can be measured via a variety of approaches. The first two that come to my mind are
to do it spectroscopically (tryptophan absorbs at 280nm) or by knowning how
much tryptophan youÕd put into the water originally, and measuring the final
amount by drying the water and weighing it.