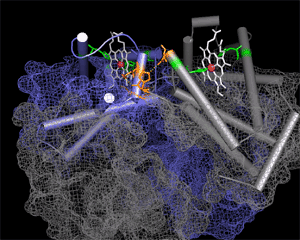
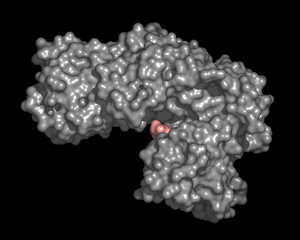
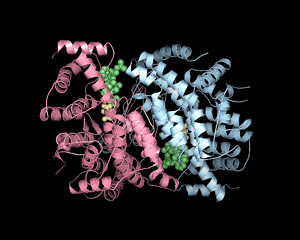
Experimental evidence suggests that flexibility and internal motions are important for many protein functions. For example, according to the induced fit hypothesis, the conformation of the enzyme changes during the substrate binding process such that interactions between the enzyme and the substrate are optimized. Induced fit is observed in many enzymes, such as hexokinase and lactate dehydrogenase. The role of conformational changes is also well understood in processes such as cooperative binding of oxygen to hemoglobin and allosteric regulation of glycogen phosphorylase and aspartate transcarbamoylase.
Traditionally, the dynamics has been illustrated by presenting and discussing the two endpoints of the dynamic process, such as the open and closed condormation of hexokinase. The dynamics can be better illustrated by movies displaying structures in motion. In our presentations, the motion effect is obtained by generating a set of interpolated structures using three-dimensional morphing of experimental atomic coordinates. It should be noted that while this produces a reasonable path from on structure to another, the path that real molecules take may differ from the predicted path. Currently, fast X-ray and dynamic NMR allows experimental study of protein dynamics in the time regime where large-scale conformational changes occur.
| Hemoglobin | Hexokinase | Citrate Synthase |
|---|---|---|
 |
 |
 |
This page was last updated March 15, 2004 and is far from complete. Return to the main page.